|
1. Introduction
The red
coloration found in bromeliads, cranberrries, carnivorous
plants, etc., is caused by plant pigments known as
anthocyanins. Because of the interest created in pigment-free
forms of certain carnivorous plants, Barry Meyers-Rice (UC-Davis),
the editor of The Carnivorous Plant Newsletter, asked me to
put together a brief summary of the science of these pigments.
While somewhat technical, it's a fascinating topic, especially
in the areas of evolution and biology. If you're interested in
learning more about this topic, read on!
(This is a modified draft of the paper submitted to Barry for
publication in the September 1998 issue of his Newsletter.)
2. Biology
Anthocyanins
are members of a class of nearly universal, water-soluble,
terrestrial plant pigments that can be classified chemically
as both flavonoid and phenolic. They are found in most land
plants, with the exception of the cacti and the group
containing the beet. They contribute colors to flowers and
other plant parts ranging from shades of red through crimson
and blue to purple, including yellow and colorless. (Every
color but green has been recorded).
Anthocyanins apparently play a
major role in two very different plant processes: for one,
attracting insects for the purpose of pollination. Advantage
is made of the fact that the pigments absorb strongly in the
UV (ultraviolet), visually attracting insects but with light
wavelengths that are invisible to humans. These pigments play
a major role in plant pollination - and in predation in
carnivorous plants, attracting insects into the trap
apparatus. (Anthocyanins play a very versatile role in
pollination, especially in the Bromeliaceae. Certain
bromeliads turn a vivid red just before and during pollination
but soon revert to the original green color characteristic of
the photosynthesis pigment, chlorophyll. Anthocyanins are not
a biochemical dead end but rather a dynamic signalling device
that can be switched on when needed by the plant to assist in
pollination. They are then degraded by plant enzymes when no
longer needed to attract pollinators to flowers.)
In their second major role,
anthocyanin-related pigments serve as a UV screen and are
produced in response to exposure of the plant to UV radiation,
protecting the plant's DNA from damage by sunlight. (UV causes
the paired strands of genetic material in the DNA double helix
to become cross-linked, preventing cell division and other
vital cellular processes like protein production).
And in a third, and no less
significant role, anthocyanins serve as anti-feedents, their
disagreeable taste serving to deter predatory animals.
In a related defense mechanism, anthocyanin production can be
induced by ionizing radiation, which can damage DNA as readily
as UV can. Chemical messengers apparently signal the damage to
DNA and induce anthocyanin production in these plants.
The biosynthesis of this class
of pigment is accomplished by a series of enzymes that are
bound to cell membranes and that help convert two central
biochemical building blocks derived from photosynthesis
(acetic acid and the amino acid phenylalanine) found in the
cell's cytoplasm through a series of discrete chemical steps
into the final pigments, which are then excreted on the other
side of the membrane into vacuoles in the epidermal cell
layer. Significant genetic change in the DNA coding for the
production of these enzymes results in loss of pigment
production.
Anthocyanin pigments can be
produced by growing plant cells in tissue culture. Plants
having no pigmentation themselves in cultivation were
subsequently demonstrated to produce anthocyanin in tissue
culture.
Environmental factors affecting
anthocyanin production included light (intensity and
wavelength, with blue and UV being most effective),
temperature, water and carbohydrate levels, and the
concentrations of the elements nitrogen, phosphorous and boron
in the growth medium. Anthocyanin production can be induced by
light, blue being the most effective color. Low light levels
also induce the formation of different flavonoid pigments,
which is another interesting adaptive response on the part of
plants. (Tillandsias, for example, develop a bright red
coloration due to induced anthocyanin production if grown in
strong light. For some additional observations on possible
alternate roles for anthocyanin in Tillandsia, see noted
bromeliad expert David Benzing's personal observations as
quoted in Paul T. Isley III's excellent book Tillandsia .)
3. Evolution
Anthocyanin-type
pigments are found only in terrestrial plants. They are not
found in animals, marine plants or in microorganisms. It is
theorized that anthocyanin production is an evolutionary
response to plants first venturing onto the stark primordial
landscape under intense UV radiation. (Significant screening
of the earth's surface from the effects of UV radiation didn't
occur until after the advent of terrestrial plants. Oxygen in
large amounts first had to be generated by the photosynthesis
of land plants before the UV-screening ozone layer was
formed).
The evolution of insect vision
to respond to the unique wavelengths of light presented by
flowering plants is an interesting scenario, as is the
evolution of these plants to take advantage of the insect's
attraction to the sight of anthocyanins. Obviously, the plants
came first and developed anthocyanins as a defense mechanism
long before the first insect evolved. Flowering plants
subsequently found in anthocyanin a handy way to attract
pollinators. Carnivorous plants took advantage of the
pollination attraction mechanism to serve as an effective
visual lure for their prey.
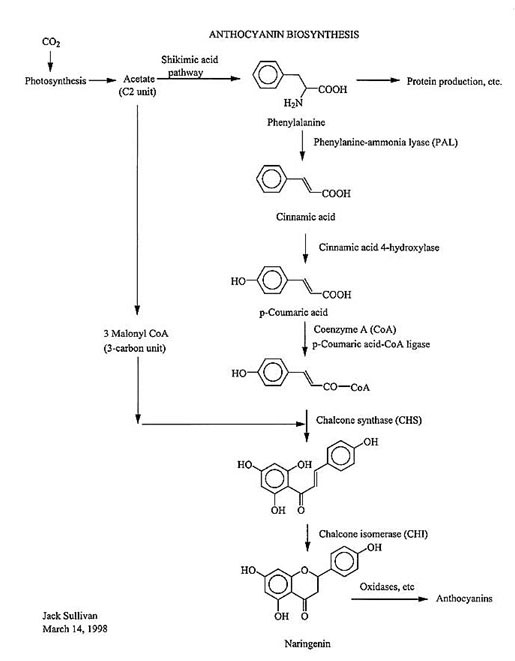
4. Chemistry
Anthocyanin
pigments are assembled from two different streams of chemical
raw materials in the cell: both starting from the C2 unit
acetate (or acetic acid) derived from photosynthesis, one
stream involves the shikimic acid pathway to produce the amino
acid phenylalanine. The other stream (the acetic acid pathway)
produces 3 molecules of malonyl-Coenzyme A, a C3 unit. These
streams meet and are coupled together by the enzyme chalcone
synthase (CHS), which forms an intermediate chalcone via a
polyketide folding mechanism that is commonly found in plants.
The chalcone is subsequently isomerized by the enzyme chalcone
isomerase (CHI) to the prototype pigment naringenin, which is
subsequently oxidized by enzymes like flavonoid hydroxylase
and coupled to sugar molecules by enzymes like UDP-O-glucosyl
transferase to yield the final anthocyanins. More than five
enzymes are thus required to synthesize these pigments, each
working in concert. Any even minor disruption in any of the
mechanism of these enzymes by either genetic or environmental
factors would halt anthocyanin production.
Anthocyanin production was
used as a visual marker in early studies of chemotaxonomy,
which studies the relationships of organisms based on their
biochemical constituents. It gave support to the one gene-one
enzyme theory that is a central tenet in the field of
molecular biology.
BIBLIOGRAPH
1. Secondary Metabolism
, by
J. Mann. 2nd edition, pp. 275-285. Oxford Univ. Press, 1987.
ISBN 0-19-855529-6
2. Secondary Plant Products
, ed. E.A. Bell
and B.V. Charwood. Encyclopedia of Plant Physiology, New
Series, vol. 8, pp. 340-349. Springer-Verlag, 1980. ISBN
0-387-09461-X
3. Natural Product Chemistry
, by Kurt B.G.
Torssell, pp. 138-145. John Wiley & Sons, 1983. ISBN
0-471-10378-0 (paperbk).
4. Plant Cell and Tissue Culture
, by J.
Reinert and M. M. Yeoman. pp. 48-50 (Experiment 13: Callus
Formaton and Anthocyanin Production in Cultures of Haplopaapus
gracilis.) Springer Verlag, 1982. ISBN 3-540-11316-9
5. Tillandsia , by Paul T. Isley III, p. 92.
Botanical Press, 1987. ISBN 0-9617675-0-2.
|